Table of contents
A. Nutrition Tables – Differences
- Reference Daily Values
- Reference Amounts Customarily Consumed (RACCs) for Serving Sizes
- Rounding Values Rules
- Nutrition Table Layout and Content
B. Product & Ingredients Names
C. Ingredients Standards of Identity
D. Permitted Food Additives & Maximum Level of Use
I. Manufacturer’s or Distributor’s Information & Country of Origin
Canada and the US made a number of changes to the food regulations in 2016, and more changes have been implemented in 2021-2022.
There are major updates, including the Canadian requirement, with an implementation deadline of December 31, 2025, of a front-of-packaging symbol, for any food product containing a high level of fat, sugar or sodium (usually 15% of the daily values of one of these nutrients).
In this article, we’ll explore the differences between U.S. and Canada food labels based on the latest regulations (2022). It is an important topic because you could be tempted to believe that, as a U.S. food manufacturer, you can simply export your product to Canada. Well, you would be wrong. Even if food labels seem to be more and more harmonized between the two countries, there are still some differences.
A. Nutrition Tables – Differences
-
Reference Daily Values
- As you know, Reference Daily Values for nutrients is the recommended amount of a given nutrient to intake per day. Note that you still find outdated documents online, even on the official FDA website. Make sure to check the date of the documentation you read. As described in our article, regulations changed in 2016 then in 2022.
- Daily values are similar for both countries, except for these 2 ingredients. Note that these values change with time (changes in 2016 then 2022).
| Daily Values For Adults | USA |
Canada |
| Fat | 78 g |
75 g |
| Potassium | 4,700 mg |
3,400 mg |
-
Reference Amounts Customarily Consumed (RACCs) for serving sizes
Both countries have defined reference amounts for different categories of foods. These represent the amount of food typically consumed in one sitting. They are rather important as they serve to determine the serving size shown in the nutrition facts tables. However, to make things even a bit more complex, Reference Amounts are related to serving sizes, but not necessarily exactly the same.
Importantly, they may be identical in Canada and the U.S., but they may be different. Let’s mention a few examples:
| Reference Amount (RACC) | USA (Oct 2018) | Canada (Oct 2024) |
| Ice cream | 2/3 cup | ¾ cup |
| Yogurt | 170 g | 115 g |
| Cottage cheese | 110 g | 125 g |
| Sugar | 8 g | 4 g |
These are just some examples to highlight differences. Note that often, but not always, the U.S. RACC is higher. Many other products will have identical RACCs, for instance, cheese, etc.
Also note that the RACCs are sometimes expressed in a different unit, for instance, “salad dressing” or “BBQ sauce” expressed in mL in Canada and grams in the USA.
If you order your nutrition labels from a company, such as FoodLab for U.S. labels, they will use such RACCs to define the correct serving size for your nutrition table.
For the complete list of Reference Amounts Customarily Consumed (RACCs), visit:
- FDA’s CFR Title 21 – Reference amounts customarily consumed
- FDA Reference Amounts Customarily Consumed: List of Products for Each Product Category: Guidance for Industry (pdf)
- Health Canada – Table Reference Amounts for Food
-
Rounding values rules
Let’s quickly mention that when creating those nutrition tables, there are specific rules on the rounding of numbers for the weight of nutrients as well as the % of daily values. Rounding is different depending on the nutrient and the amount of each nutrient.
-
Nutrition table layout and content
Let’s compare the two nutrition tables side-by-side (current to 2024): 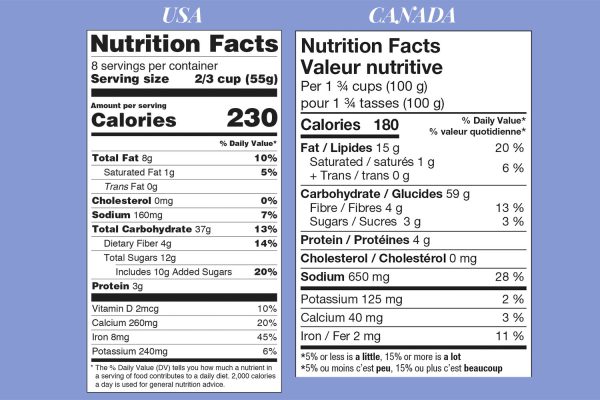
- Unless you’re a local Canadian product outside Québec, your nutrition table must be bilingual (or you must have a separate table in each language),
- Font sizes are very different, in particular, the U.S. label emphasizes serving size and calories,
- The order of nutrients differs,
- Only the U.S. label lists the Daily Value % for cholesterol,
- The U.S. label specifies the amount of added sugars,
- The U.S. label includes Vitamin D,
- The explanatory text below the table differs.
STRUGGLING WITH CANADIAN FOOD LABELING COMPLIANCE?
Whether it’s ingredient standards of identity, front-of-package symbol, bilingual labelling, or nutrition facts, we’re here to help you navigate the process with confidence.
A quick call is the first step toward compliance success!
B. Product & Ingredients Names
It is important to realize that there are a number of products on grocery shelves in Canada, with non-compliant names. As a food manufacturer, you cannot trust other products labeling, when designing your own.
The Canadian Food Inspection Agency (CFIA), responsible for implementing food labeling regulations, has strict requirements regarding the naming of products. Product names should be checked versus what are the acceptable common names (product names) for a given food. And names need to represent clearly, precisely and accurately what the food is, to avoid consumer confusion.
As an example, you cannot interchangeably call a mix a spice mix, or herbs or seasonings. The CFIA website has the list of spices, of herbs and defines what seasonings are. If a “spice mix” contains salt, it should not be called a spice mix but a seasoning.
Then, you must ensure that the French translation is also acceptable. Number of translations are not compliant, as their meaning is misleading or sometimes totally irrelevant. I saw a label recently where the word “rub” was translated in French to “ruban”, which means “lace”. Or this one for “crushed peppers” for which the translation “poivron rompu” means “broken bell pepper”.
As you know, there are no literal translations, and that’s one of the reasons why a French name may be non-compliant. Going back to the “rub” example, a common translation is “épices à frotter” (rubbing spices). If that translation is totally acceptable in a recipe, it will not be acceptable on a food label, if the rub contains spices and salt, as a product which contains salt – is a seasoning, not a spice mix.
Food manufacturers must also refer to the Standards of Identity to properly name their products (see section C – Ingredients Standards of Identity). For instance, what is the standard of identity for a tomato paste versus a tomato puree? What is the standard of identity for a jam or for bread?
Finally, when designing the layout, don’t forget that the names must be of equivalent size in English and in French (see section H – General Layout).
C. Ingredients Standards of Identity
As a U.S. manufacturer, it is important to understand that you may need to make some changes to your ingredients when importing to Canada. Each country has established “Standards of Identity” for a certain number of foods. These standards of identity define the ingredients’ characteristics. The FDA has 300 identity standards in 20 categories of foods and these standards may differ from the Canadian Standards of identity.
For instance, in Canada, a “jam” must contain at least 45% of fruit, and cannot contain apple or rhubarb. In the USA, jam can contain apples or rhubarb. In Canada, flour must be enriched flour, i.e., it must contain a certain % of thiamine, riboflavin, niacin, folic acid, and iron per 100 grams of flour. “Bread” is a “yeast-leavened dough made of flour and water” with certain types of fat, gum, or yeast.
Finally, you must check the authorized French translation for all your ingredients. Here are the links to the U.S. and the Canadian Standards of Identity:
D. Permitted Food Additives & Maximum Level of Use
Some food additives or coloring agents may be authorized in the U.S. and not in Canada, or vice-versa.
Additionally, food additives can be permitted in some foods and not others, and also be permitted in a limited amount (maximum level of use), such amounts varying depending on the food product. You’ll also need to know the approved naming of these food additives and understand what the authorized French translation for such additives are.
Here are the links to the latest U.S. and Canadian lists of permitted food additives:
E. Allergens
If your packaging labeling doesn’t conform to the regulations, it may be the subject of a recall.
In that regard, it is crucial to list all the allergens with mandatory declarations. Allergen declaration is one of the top reasons for product recalls.
Be aware that the list of allergens with mandatory declaration is different in the U.S. and Canada.
1. Allergens on Canadian food labels
Below is the list of priority allergens which must be declared in Canada (see Health Canada reference):
-
- tree nuts (almonds, Brazil nuts, cashews, hazelnuts, macadamia nuts, pecans, pine nuts, pistachios or walnuts);
- peanuts;
- sesame seeds;
- wheat or triticale;
- eggs;
- milk;
- soy;
- crustaceans and molluscs;
- fish;
- mustard
- sulphites (Canadian spelling)
Additionally, gluten sources must be declared (barley, oats, rye, triticale, and wheat).
2. Allergens on US food labels
For the USA, a new guidance on Food Allergen Labeling Requirements (pdf) was issued on January 6, 2025.
This comprehensive guidance aims to enhance transparency in food labeling, thereby helping consumers manage food allergies more effectively.
Key updates include:
- Inclusion of Sesame as a Major Allergen: Sesame is now recognized as the 9th major food allergen in the U.S. Any food containing sesame must clearly declare its presence on the label.
- Clarifications on Allergen Labeling: The guidance offers detailed questions and answers regarding the labeling of major food allergens, including tree nuts, milk, and eggs. It addresses specific scenarios such as the labeling of dietary supplement products and the handling of incidental additives.
- Guidance on Specific Packaging and Labeling Situations: The document provides insights into labeling requirements for particular cases, such as individual units within a multi-unit package, ensuring that allergen information is consistently communicated to consumers.
Read FDA finalizes two guidance documents related to food allergens.
Below is the revised list of allergens to declare in the USA:
- Milk
- Eggs
- Fish (e.g., bass, flounder, cod)
- Crustacean shellfish (e.g., crab, lobster, shrimp)
- Tree nuts (e.g., almonds, walnuts, pecans)
- Peanuts
- Wheat
- Soybeans
- Sesame
Gluten & sulfites: As per FDA communications, “Gluten, certain additives (for example, yellow 5, carmine, sulfites), and other food allergens for which new science has emerged, are examples of other substances the FDA monitors and, in some cases, requires specific labeling for.“
As you can see, Canada requires mustard seeds to be declared, whereas it is not an allergen with mandatory declaration in the USA. Additionally, Canada is more strict regarding allergen cross-contamination.
STRUGGLING WITH CANADIAN FOOD LABELING COMPLIANCE?
Whether it’s ingredient standards of identity, front-of-package symbol, bilingual labelling, or nutrition facts, we’re here to help you navigate the process with confidence.
A quick call is the first step toward compliance success!
F. Health Claims
If you make any health claim on your packaging, you must make sure that these are acceptable in the other country when you decide to export.
Health claims include whole grains, organic, gluten-free, natural, non-GMO, etc.
Here are a few examples of things to consider:
- Make sure that your “grains” are “whole grains.” One of my clients’ products contained masa (a dough made from corn flour and used to make tortillas.) Regulators in Canada do not allow masa to count as a whole grain. So she had a “whole grain” claim on her U.S. packaging but could not have such a claim on her Canadian label. Another customer had a “whole grain” claim but used white rice. Again, she could not have such a claim in Canada.
-
 Check your organic claims with your USDA-Accredited Certifying Agent to confirm that your product can be certified “organic” in Canada. Your USDA organic certification must be COR (Canadian Organic Regime) equivalent. “The U.S. has an “equivalency arrangement” with Canada. It means that as long as the terms of the equivalence arrangement are met, organic operations certified to the USDA organic regulations or Canada Organic Regime may be labeled and sold as organic in both countries”. The important words here are: “As long as the terms of the equivalence arrangement are met.” There are additional production requirements for U.S. products to be certified organic in Canada, and vice-versa.
Check your organic claims with your USDA-Accredited Certifying Agent to confirm that your product can be certified “organic” in Canada. Your USDA organic certification must be COR (Canadian Organic Regime) equivalent. “The U.S. has an “equivalency arrangement” with Canada. It means that as long as the terms of the equivalence arrangement are met, organic operations certified to the USDA organic regulations or Canada Organic Regime may be labeled and sold as organic in both countries”. The important words here are: “As long as the terms of the equivalence arrangement are met.” There are additional production requirements for U.S. products to be certified organic in Canada, and vice-versa.
More information at International Trade Policies: Canada – US – Organic Standards. Note that you can either use the USDA organic logo on your Canadian packaging or the Canadian organic logo. It seems that the USDA organic logo is more commonly used. There are also requirements regarding the mention of your certification body.  Understand the non-GMO regulations in both countries, as it requires specific wording on your labels, which is different in the U.S. and Canada. In particular, it is essential to understand the difference between genetically-modified and genetically-engineered.
Understand the non-GMO regulations in both countries, as it requires specific wording on your labels, which is different in the U.S. and Canada. In particular, it is essential to understand the difference between genetically-modified and genetically-engineered.
G. Front-Of-Packaging Symbol
Read our complete article on the New Front-Of-Packaging Nutrition Regulation.
H. Net Quantity Statement
Net quantity is expressed/written differently in the U.S. versus Canada, and, as mentioned above, the unit can be different, e.g. “salad dressing” or “BBQ sauce” expressed in mL in Canada and grams in the USA.
I. Manufacturer’s or Distributor’s Information & Country of Origin
Canada and the U.S. also have specific instructions regarding the name and address information, as well as the country of origin. The information relates to the responsible party for the imported products, i.e. either the foreign manufacturer or the Canadian distributor.
J. “Best before,” “Package on” and Storage Information
In Canada, there are strict guidelines on expiry and storage information, including the phrasing, the date formatting and the location of the information on the label. Note that the requirements are different for products with a “life” of less than 90 days than for food with longer shelf life.
K. General Layout
There is indeed a long list of rules to follow on:
- the font size of various elements on the package,
- the location of the elements,
- the borders around information,
- the background color for ingredients and nutrition tables,
- the size of nutrition tables depending on the overall package size (not the label size).
Some of those rules have recently changed. They would be too long to list here, but it highlights the importance of working with the right label specialist and graphic designer.
We included several key reference documents here, to help you navigate the field of food labeling regulations in Canada and the U.S.
However, we haven’t covered all the challenges you may encounter, as each food product has its own unique requirements. It’s a complex subject.
Additionally, typographic rules differ between English and French, making it essential to work with a graphic designer who understands these nuances.
Need American or Canadian-compliant food labels, compliant with the new 2022 regulations? Need some field research for your food products? Feel free to contact us. We can help.

When exporting products from canada to USA, which Nutrition fact chart is to be used on the labels.
Bonjour,
Je souhaite importer des produits aux USA et au Canada, j’ai des besoins d’étiquetages alimentaires conformes aux normes américaines ou canadiennes, conformes à la nouvelle réglementation de 2016.
Merci pour votre aide.
When exporting products from Canada to the USA, you must use the US nutrition fact tables.
Bonjour, N’hésitez pas à me contacter par courriel avec des informations détaillées sur vos produits et vos besoins. Cordialement.