UPDATE: We are currently writing an article on the 2022 new Food Labeling Regulations for both the USA and Canada. There are major updates, including the Canadian requirement, with an implementation deadline of December 31, 2025, of a front-of-packaging symbol, for any food product containing a high level of fat, sugar or sodium (usually 15% of the daily values of one of these nutrients).
This article is an overview of the compliance guide about the new 2016 Nutrition Facts Labels, issued by the FDA for the small food businesses in January 2020. The goal is to help you have a strong understanding of the elements that changed. We recommend that you refer to the compliance guide itself for any specific topic of interest. The pdf starts with a table of contents where you can click on any section title and go directly to that section.
You can find this guide at: “Food Labeling: Revision of the Nutrition and Supplement Facts Labels: Guidance for Industry – Small Entity Compliance Guide“ (pdf).
For small food businesses, i.e., food manufacturers with less than $10 million in annual food sales, the compliance date to implement the new 2016 Nutrition Facts labels is January 1, 2021.
For single-ingredient packages and/or containers of pure honey, pure maple syrup, or other pure sugars and syrups, as well as the cranberry products, the FDA intends to exercise enforcement discretion until July 1, 2021.
This article will cover the same chapters:
- Which foods do the new rules cover?
- Nutrients declaration: Which nutrients? What are the changes?
- Which records are necessary?
- Changes in the values of nutrients declaration,
- What are the changes in format requirements?
- Are the Nutrition Facts requirements for foods in small packages?
- What are the requirements for Supplement Facts labels?
A. Which foods do the new rules cover?
All foods which have nutrition labeling requirements. i.e., not exempt from nutrition labeling requirements or subject to special labeling requirements.
B. Nutrients declaration: Which nutrients? What are the changes?
-
Which new nutrients must be declared?
- Added Sugars: This includes sugars from syrups, honey, maple syrup, and some sugars from fruit or juice concentrate (see details in the guide (pdf).) There are some specific considerations for single-ingredient sugars and syrups (see “The Declaration of Added Sugars on Honey, Maple Syrup, Other Single-Ingredient Sugars and Syrups, and Certain Cranberry Products” (pdf)).
- Vitamin D: The weight and percentage of daily value must be declared, just above calcium.
- Potassium: The weight and percentage of daily value must be declared, just after iron.
-
How to calculate added sugars
A specific FDA guide explains how to calculate the weight of added sugars and which ingredients to take into consideration in the calculation: “Nutrition and Supplement Facts Labels: Questions and Answers Related to the Compliance Date, Added Sugars, and Declaration of Quantitative Amounts of Vitamins and Minerals“ (pdf).
-
What are the changes to the definition or presentation of nutrients previously declared?
These can be changes in the definition or the presentation of these nutrients.
-
- Dietary fiber: The definition of dietary fiber is “non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health.”
- Total sugars: “Sugars” was changed to “Total Sugars,” which includes both “Added Sugars” and sugars that are naturally occurring in food. The declaration of “Total Sugars” is not required if the product contains less than one gram of sugars per serving and no claim is made about sugars, sweeteners, or sugar alcohol. In this case, the statement “Not a significant source of total sugars” must be added at the bottom of the table.
- Sugar Alcohol: Sugar Alcohol must be declared when a claim is made about added sugars and it is present.
See below, some changes in the values of nutrients.
-
Which isolated or synthetic non-digestible carbohydrates should you include in the calculation of dietary fiber?
Check the latest FDA information online, as the FDA is reviewing some isolated or synthetic non-digestible carbohydrates to include in the calculation of the amount of dietary fiber. Read: “What isolated or synthetic fibers have FDA included in its dietary fiber definition?“
-
How to calculate dietary fiber
You may use an AOAC method of analysis (AOAC 2009.01, AOAC 2011.25, or an equivalent) to measure the amount of dietary fiber in a serving of a product.
-
Which nutrients can be voluntarily declared?
The following nutrients can be voluntarily declared. However, a declaration is mandatory when a claim is made.
-
- Vitamins C and A,
- Fluoride,
-
Which nutrients are no longer declared?
The following declarations are no longer allowed:
-
- Calories from fat,
- Total carbohydrates,
C. Which records are necessary?
Records are necessary for the amount of:
- Added sugars, when both added sugars are naturally-occurring sugars are present,
- Added non-digestible carbohydrates which do not meet the definition of dietary fiber, in certain cases (see guidance for details),
- Added rac-α-tocopherol and RRR-α-tocopherol, when both present,
- Synthetic folate and/or folic acid and naturally-occurring folate, when both present.
Records can be analyses of databases, recipes or formulations, or batch records. Records must be kept for two years after the food product is marketed.
D. Changes in the values of nutrients declaration
-
What are the changes to the values of nutrients declared?
- Changes in the calculation of caloric content – read the guidance for details (pdf),
- Updated Daily Reference Values (DRVs) – see figure 1 below,
- Updated Reference Daily Intakes (RDIs) – see figure 2 below.
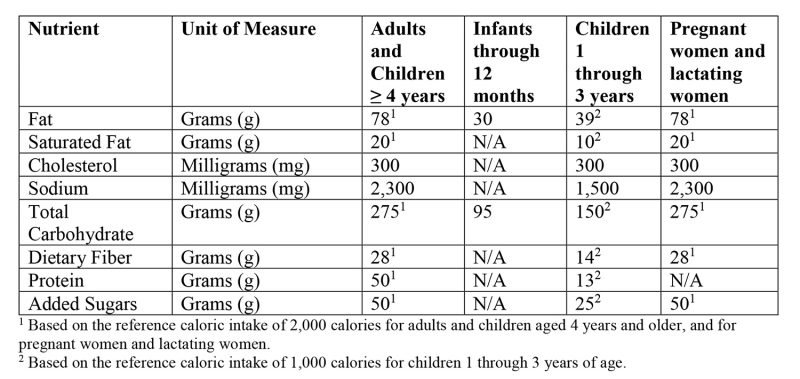
Figure 1: Updated Daily Reference Values (DRVs) 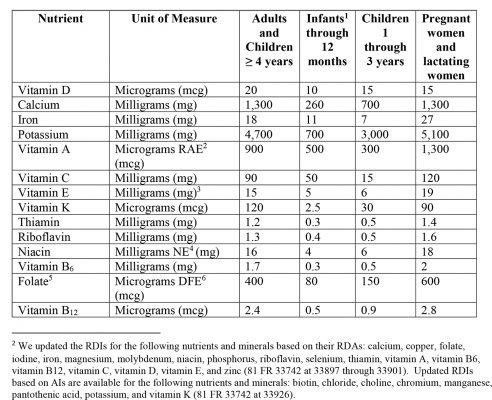
Figure 2: Updated Reference Daily Intakes (RDIs) 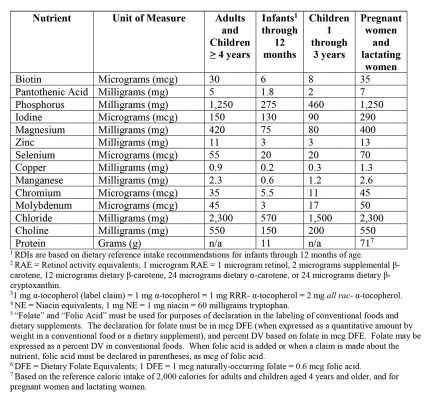
Figure 2 (cont’d): Updated Reference Daily Intakes (RDIs)
-
What are the changes to the units of measure?
The units of measure have changed for vitamin A, vitamin D, vitamin E to a metric unit of measure. Folate must be declared in mcg Dietary Folate Equivalents (DFE). Niacin unit of measure is changed from “mg” to “milligrams NE.”
-
Which vitamins and minerals are mandatory to include?
It is mandatory to declare vitamin D, calcium, iron, and potassium.
-
How to present vitamins and minerals
Both absolute amounts (the quantitative amount by weight) and percent daily value (DV) must be declared. However, if the amount less than 2% of the Reference Daily Intake (RDI), it can be indicated with a zero or an asterisk, and with a note below the table “Contains less than 2 percent of Daily Value of this (these) nutrient (nutrients).”
Alternatively, the declaration is not required if the statement “Not a significant source of (listing the vitamins or minerals omitted)” is placed at the bottom of the table.
For any other nutrient added as a nutrient supplement or when a claim is made, the declaration must include the “% of daily value (DV).”
Some rounding recommendations are explained in FDA’s guidance entitled “Nutrition and Supplement Facts Labels: Questions and Answers Related to the Compliance Date, Added Sugars, and Declaration of Quantitative Amounts of Vitamins and Minerals.”
-
How do the nutrient requirements for supplements differ?
The FDA updated the Supplement Facts label to correspond to the Nutrition Facts label. However, there are still some differences between the two (see guidance for details).
-
Has nutrient compliance or the level of variance allowed changed?
The updated rule does not change the level of variances allowed, but now it also includes soluble fiber and insoluble fiber and added sugars.
-
Which version of the official methods of analysis of the AOAC International should you use?
The final rule references the Official Methods of Analysis of the AOAC International, 19th edition (2012).
E. What are the changes in format requirements?
The FDA strongly recommends using the following graphic specifications for Nutrition and Supplement Facts labels.
-
How to comply with the requirements for the new standard vertical version of the Nutrition Facts Label
The standard vertical format (see figures 3 to 5 below, p. 21-22 of the guidance) must be used, except when another format (tabular, etc.) is permitted.

Figure 3: Standard vertical with mandatory nutrients
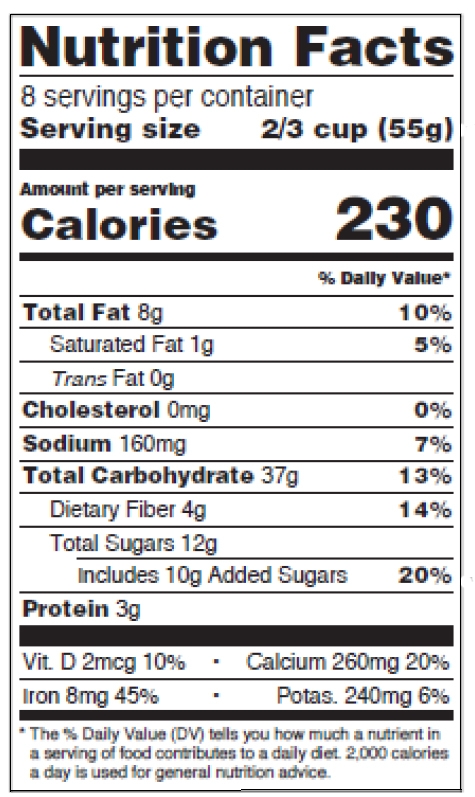
Figure 4: Standard vertical, side-by-side
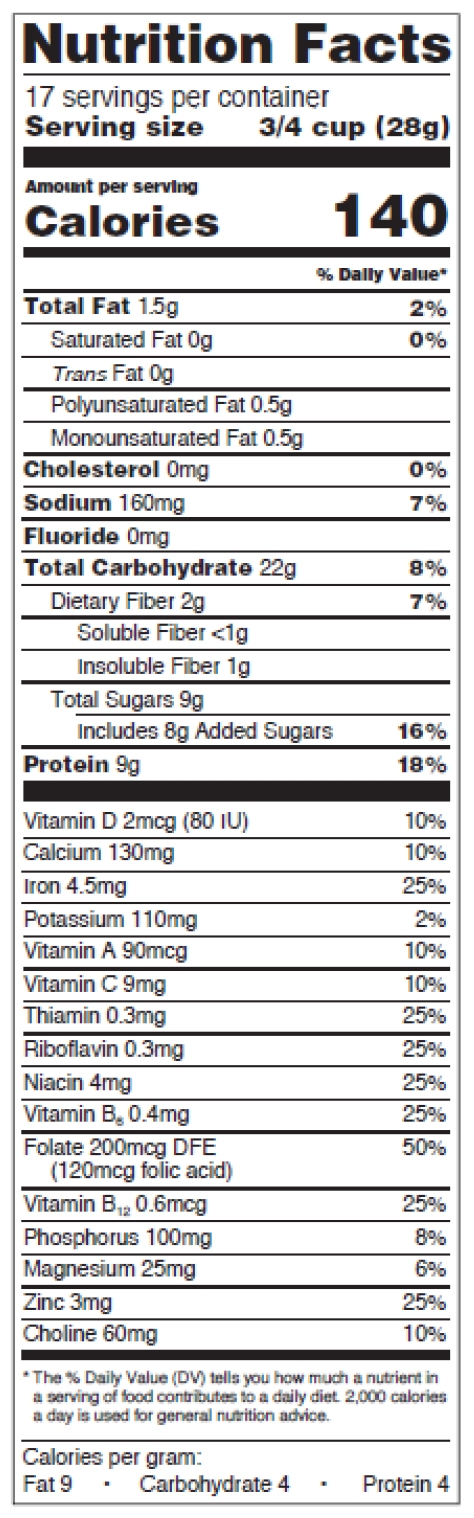
Figure 5: Standard vertical, mandatory and voluntary nutrients
The format requirements for the new standard vertical version are:
-
- Header “Nutrition Facts” set to full width and in a type size no smaller than all other print sizes in the nutrition label, except for the numerical value for “Calories” (no smaller than 22 points),
- Thin hairline beneath the header,
- “___ servings per container” declaration immediately below the “Nutrition Facts” header, no smaller than 10 points,
- “Serving size” no smaller than 10 points, in bold or extra bold type,
- “Serving size” numerical value right-justified, allowed to be 8 points when it doesn’t fit the space,
- Type size of word “Calories” no smaller than 16 points, in bold or extra bold type,
- Type size of numerical value for “Calories” no smaller than 22 points, in bold or extra bold type,
- Use the statement “Includes ‘X’ g Added Sugars” and the % of daily value (DV) to declare added sugars, right below “Total Sugars” and indented,
- Mandatory vitamins and minerals separated from other nutrients by a bar, listed vertically or side-by-side, type size no smaller than 8 points. If listed side-by-side, list vitamin D and calcium on the 1st line,
- Change in the wording of the “% Daily Value*” footnote. It now reads: “*The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice.” You can omit the footnote on calorie-free products.
For more information on the updates made to the Reference Amounts Customarily Consumed and Serving Size regulations, read the final guidance entitled “Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed At One Eating Occasion, Reference Amounts Customarily Consumed, Serving Size-Related Issues, Dual-Column Labeling, and Miscellaneous Topics“ (pdf).
-
How to present added sugars
- Use the statement “Includes ‘X’ g Added Sugars” and the % of daily value (DV) to declare added sugars, right below “Total Sugars” and indented,
- The % Daily Value declaration is required,
- Added sugars content must be expressed to the nearest gram,
- When a serving contains less than 1 gram of added sugars:
- May use “Contains less than 1 gram” or “less than 1 gram”,
- May express as zero if the serving contains less than 0.5 gram,
- The declaration is not required if no claim is made about sweeteners, sugars, or sugar alcohol content,
- If “added sugars” is not declared, use the statement “Not a significant source of added sugars” at the bottom of the table.
-
How to comply with the Nutrition Facts Label requirements for infants and children under 3
Children’s food labeling is not our expertise, so we’d like to refer you to the guide. The requirements are explained on pages 25-26 of the pdf. See figures 6-7 below.
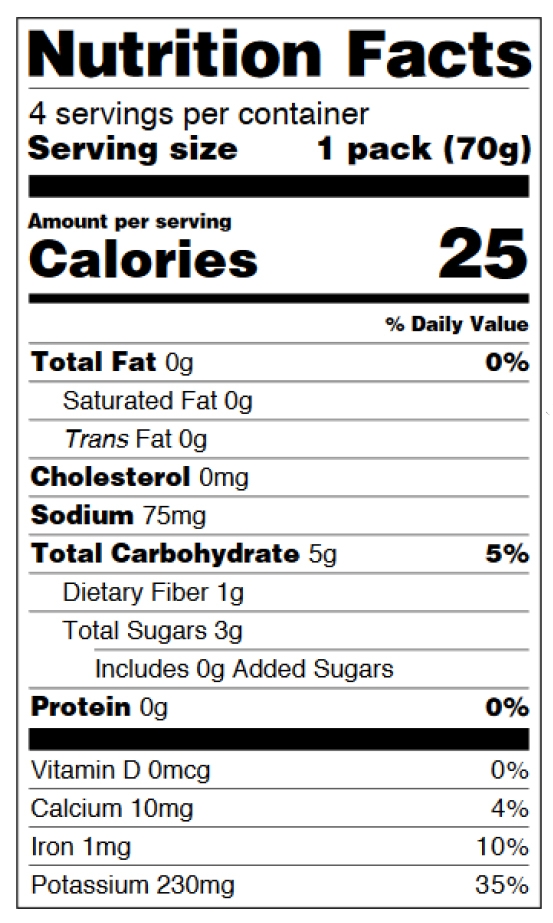
Figure 6: Infants through 12 months of age
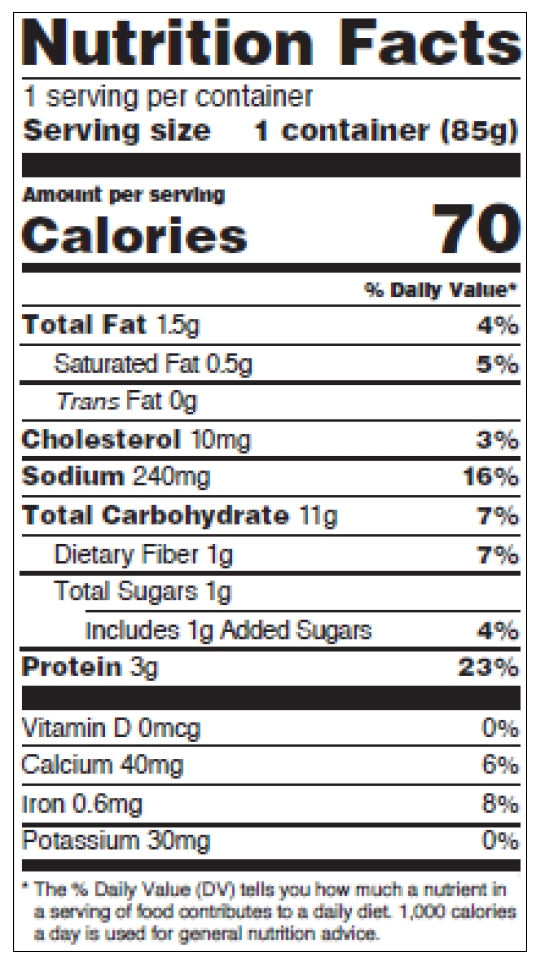
Figure 7: Children 1 through 3 years
-
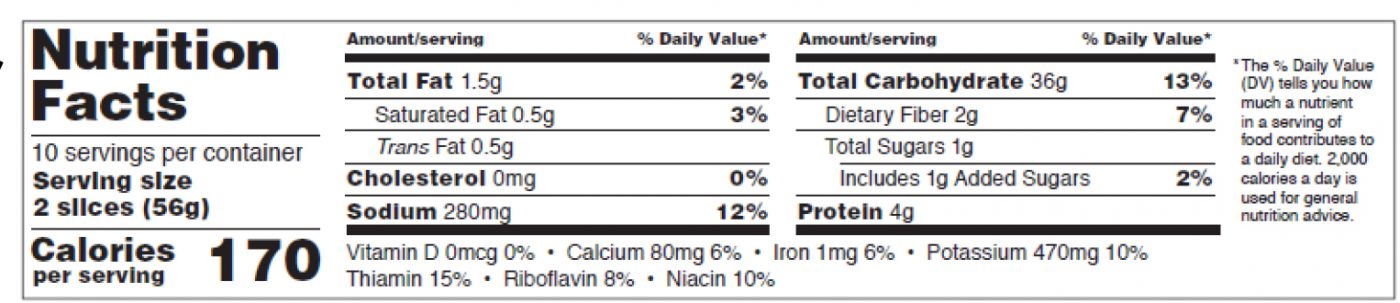
When can we use the tabular format of the Nutrition Facts Table?
You can use the tabular format (see figure 8 below, p.27 of the pdf) when there is not enough vertical space for the required components of the nutrition label. It is different from the tabular format for small or intermediate-sized packages.
The format requirements for the new tabular format are:
-
- “Nutrition Facts” header not set across the whole width of the label,
- The word “Calories” must be in a type size no smaller than 10 points,
- “Serving size” no smaller than 9 points, in bold or extra bold type.
-
How to comply with the new requirements for the Nutrition Facts Label when the total surface area available for labeling is 40 or fewer square inches?
See figure 9 below, p. 28 of the pdf: You can use the tabular display for small packages or intermediate-sized packages when you cannot accommodate either the standard vertical display (figures 3-5) or the tabular display (figure 8).
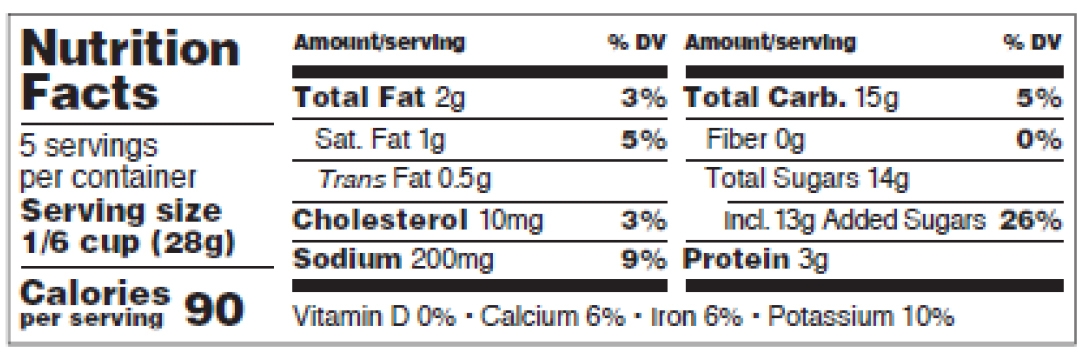
For those small packages, the linear format (figure 10 below, p. 28 of the pdf) can also be used, but only if the label cannot accommodate a tabular display.

On that linear format:
-
- The actual number of servings may be listed instead of the servings per container,
- Some abbreviations are accepted (see list p.28-29 of the pdf.)
For the small packages tabular of linear format tables (figures 9 and 10):
-
- Numeric value for “Calories” in a type size no smaller than 14 points,
- Word “Calories” in a type size no smaller than 10 points,
- “___ servings per container” in a type size no smaller than 9 points,
- “Serving size” no smaller than 9 points, in bold or extra bold type,
- The “% Daily Value*” footnote is not mandatory,
- If “Daily Value” is not spelled out in the header, an asterisk can be placed at the bottom of the label with the statement “% DV = % Daily Value” in a type size no smaller than 6 points.
-
How to comply with the new requirements for the Nutrition Facts Label when using dual-column labeling
See figures 11-15 below, p. 31-33 of the pdf.
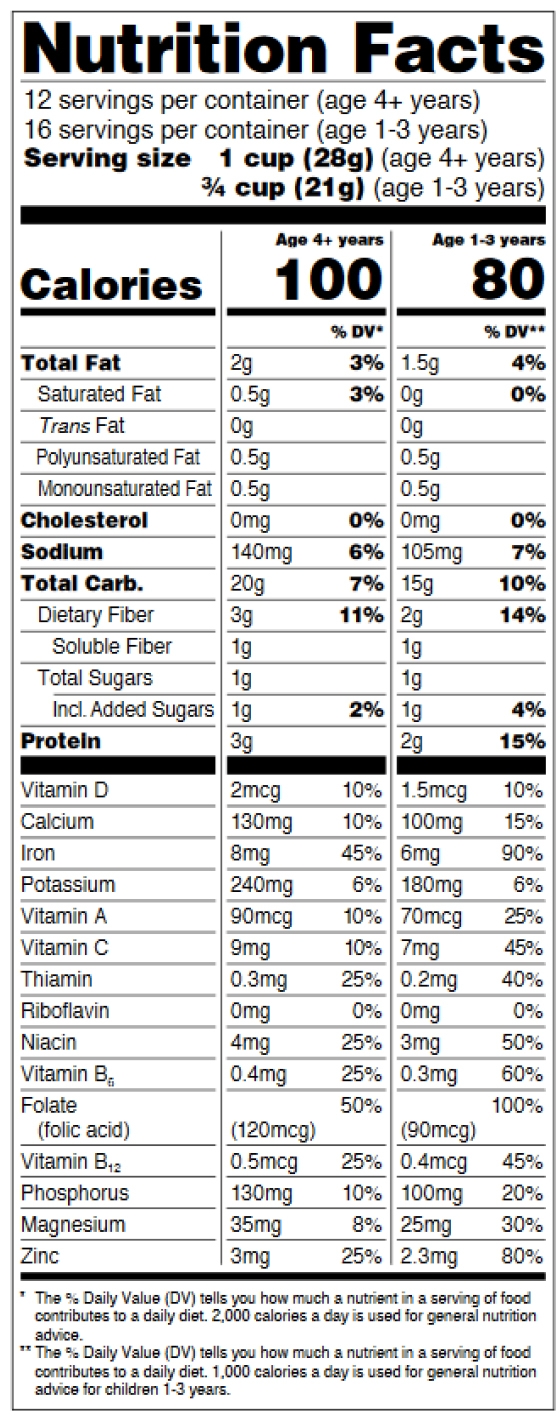
Figure 11: Dual Column Display for 2 different RDI Groups
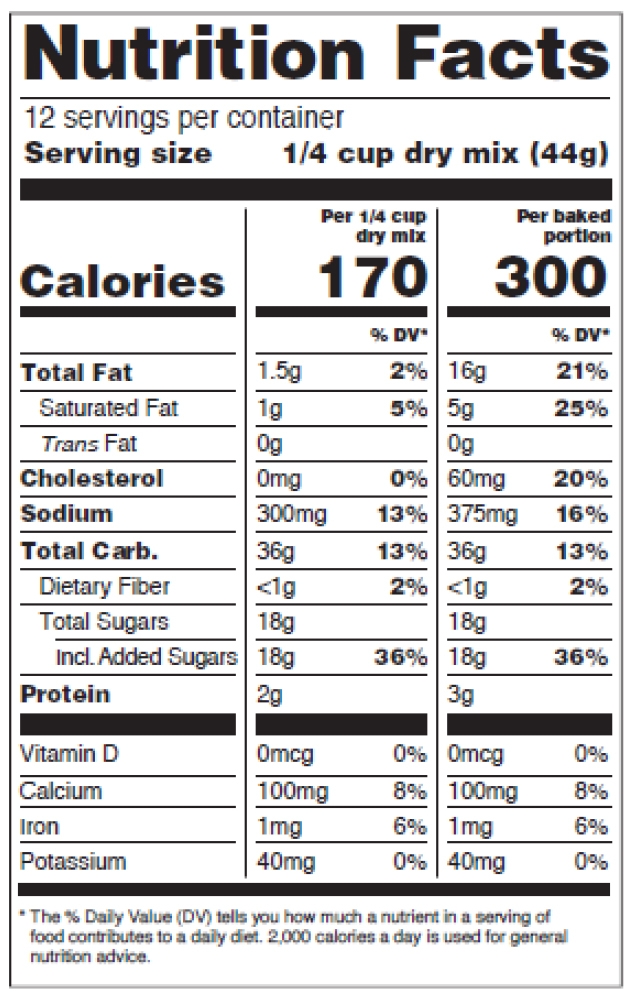
Figure 12: Dual Columns, Two Forms of the Same Food
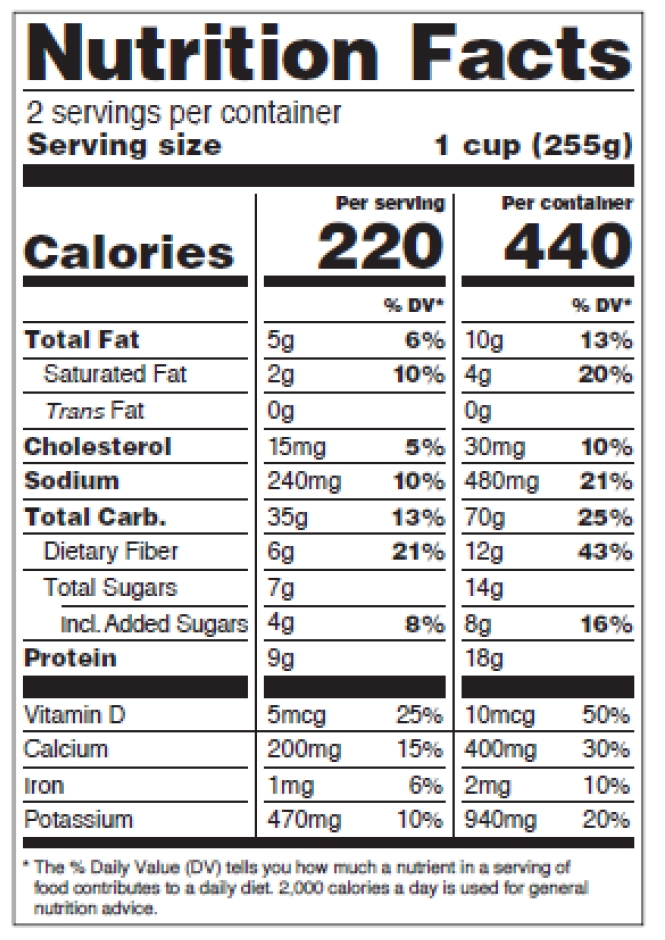
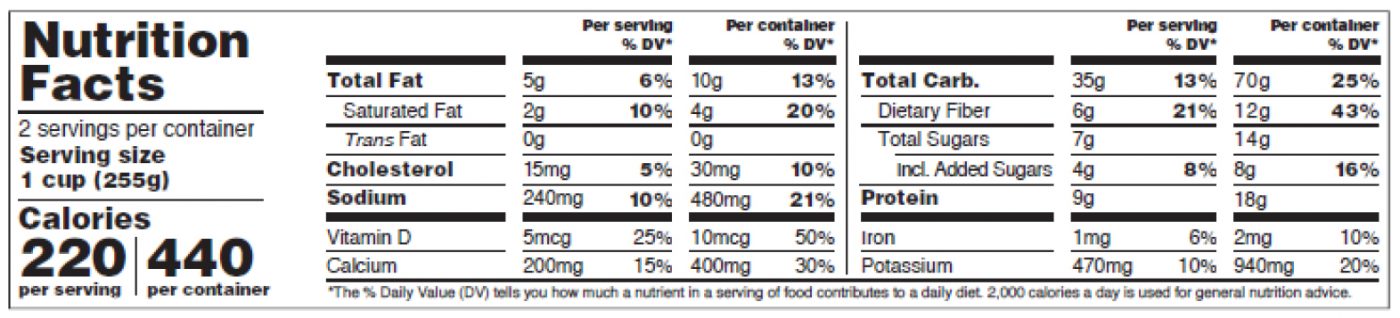
Figure 13: Dual Column Display, Per Serving and Per Container
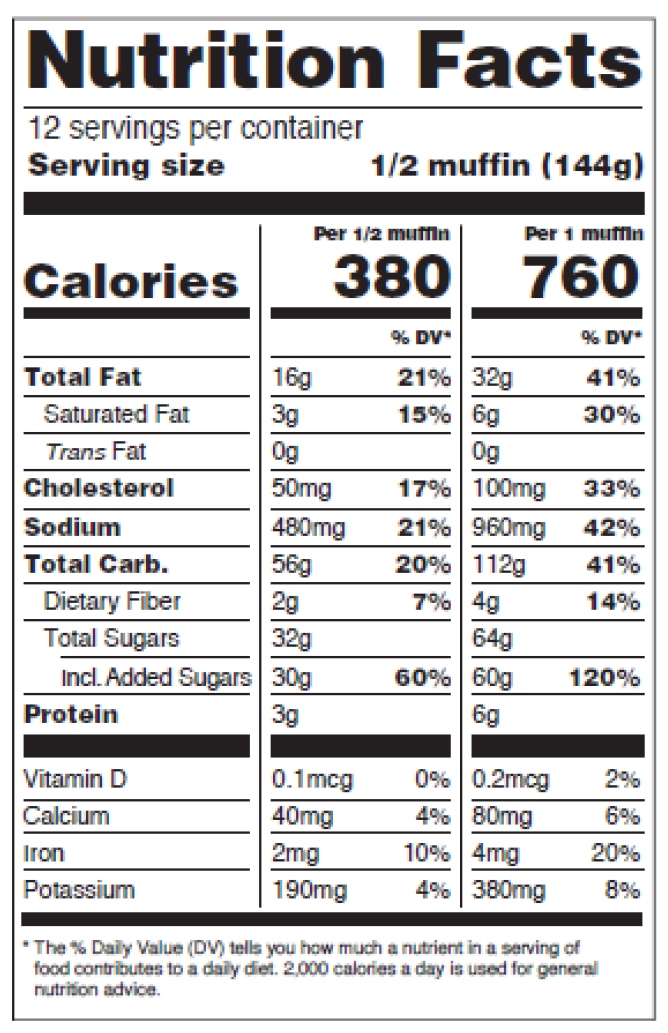
Figure 14: Dual Column Display, Per Serving and Per Unit
Dual-column labeling is required in certain situations or used voluntarily.
For more information on dual-column labeling, read the final guidance entitled “Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed At One Eating Occasion, Reference Amounts Customarily Consumed, Serving Size-Related Issues, Dual-Column Labeling, and Miscellaneous Topics” (pdf).
For dual-column nutrition facts tables:
-
- The column headers describe why the dual column display is being used,
- Weight and percent DV must be presented for each column,
- Columns must be separated by vertical lines,
- “Calories” in a type size no smaller than 10 points,
- Numeric value for “Calories” in a type size no smaller than 22 points,
- “Serving size” declaration in a type size no smaller than 9 points,
- “___ servings per container” declaration in a type size no smaller than 10 points,
- Nutrient information for vitamins and minerals must be separated from the information on other nutrients by a bar,
- Nutrient information for vitamins and minerals must be presented vertically in the required order
- The “Amount per serving” statement is not required,
- Abbreviations of “Total Carb.” and “Incl.” are allowed,
- The appropriate “% Daily Value” footnote for each population group is included.
-
When can the simplified format be used?
The simplified format (figure 16 below, p.34 of the pdf) can be used when a food product contains insignificant amounts of eight or more of the following: Calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, and potassium (and for foods intended for infants through 12 months of age and children 1 through 3 years of age, when a food product contains insignificant amounts of six or more of the following: Calories, total fat, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, or potassium.)
-
- The footnote “Not a significant source of ___,” listing all nutrients present in insignificant amounts, is required,
- The “% Daily Value” footnote is not required,
- The “% Daily Value” can be abbreviated as “% DV,” with an asterisk at the end of the abbreviation and the corresponding footnote “% DV = % Daily Value.”

-
Are the Nutrition Facts required for foods in small packages with a total surface area available for labeling of less than 12 square inches?
It is not required unless you make a nutrition claim.
If you don’t include the Nutrition Facts table, you must provide an address or telephone number so that a consumer can get the nutritional information.
-
What are the requirements for Supplement Facts labels?
Figure 17 shows the Supplement Fact label. However, this is not our expertise. We’d like to refer you to the guide. The requirements are explained on pages 35 of the pdf.

We hope this overview was useful. Let us know in the comments below if you have any questions.
Need some field research for your food products? Need American or Canadian-compliant food labels, compliant with the new 2016 regulations? Feel free to contact us. We can help.
